-
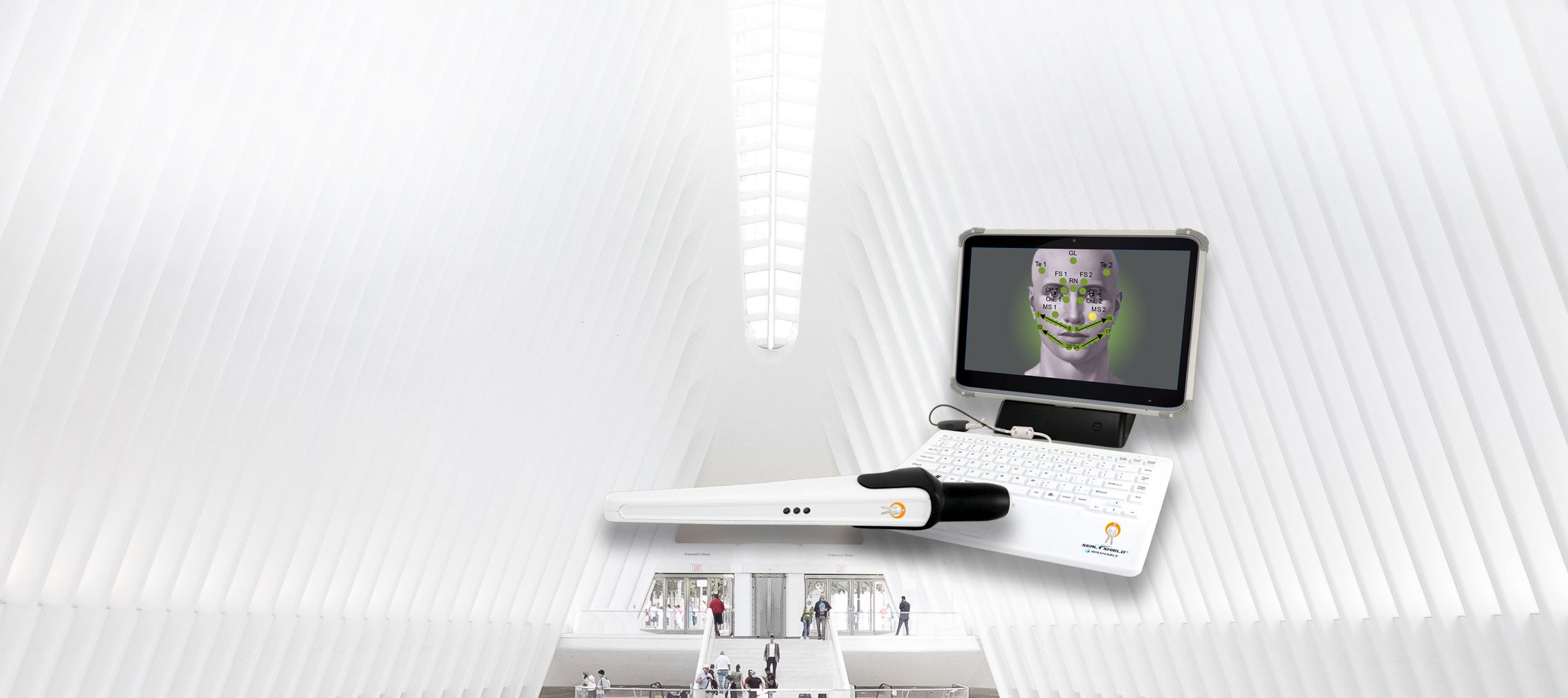 state-of-the-art health testing system
state-of-the-art health testing systemThe AlfaSight 9000TM
AlfaSight allows you to SEE ORGAN FUNCTION by utilizing
the Autonomic Nervous System’s signaling by way
of the segmental organ and tissue connections. -
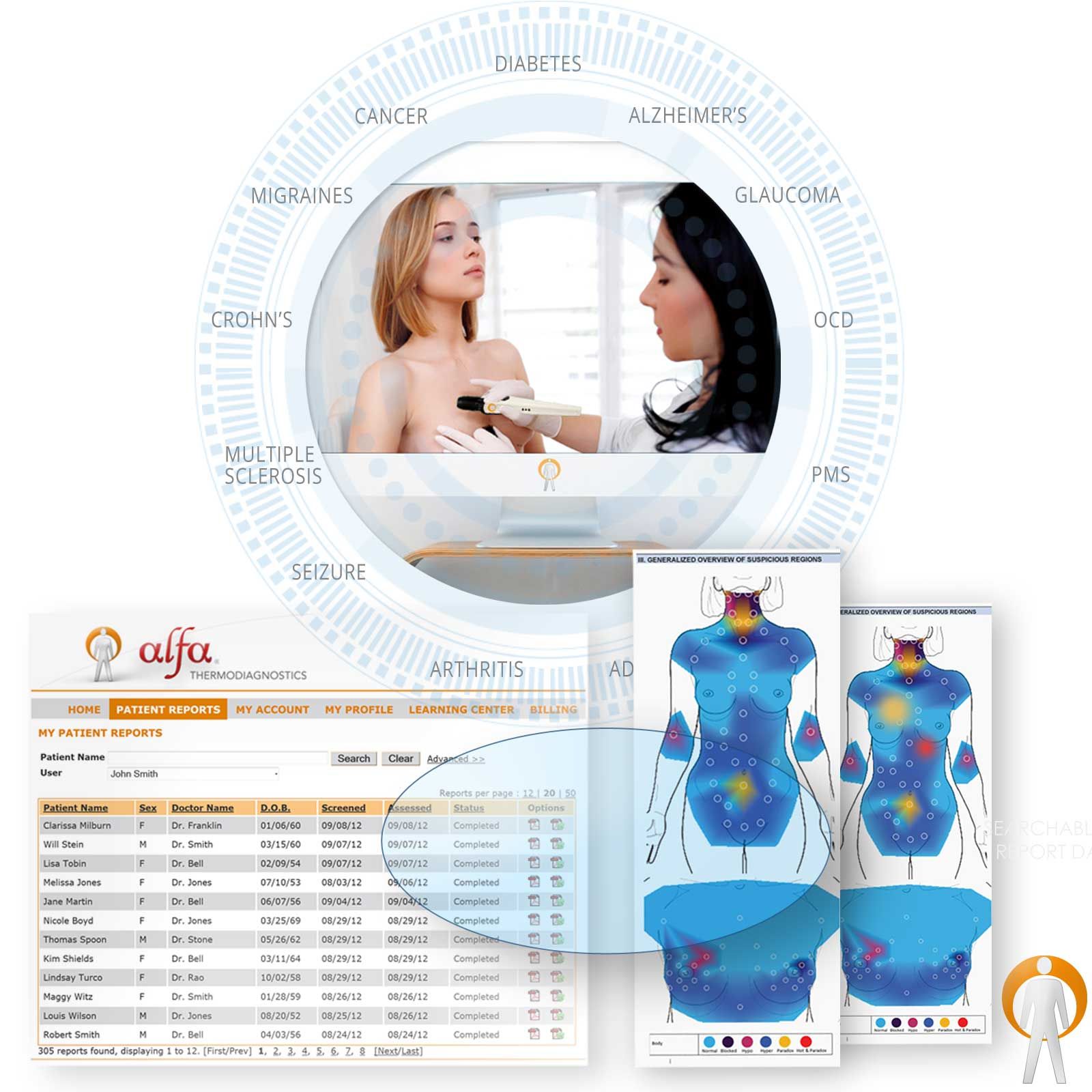
 WHOLE-BODY WELLNESS SCAN
WHOLE-BODY WELLNESS SCANDetect Early Health Warnings
Add the AlfaSight 9000 to provide
SENSITIVE DIAGNOSTICS for prediction
and comprehension of disease processes. -
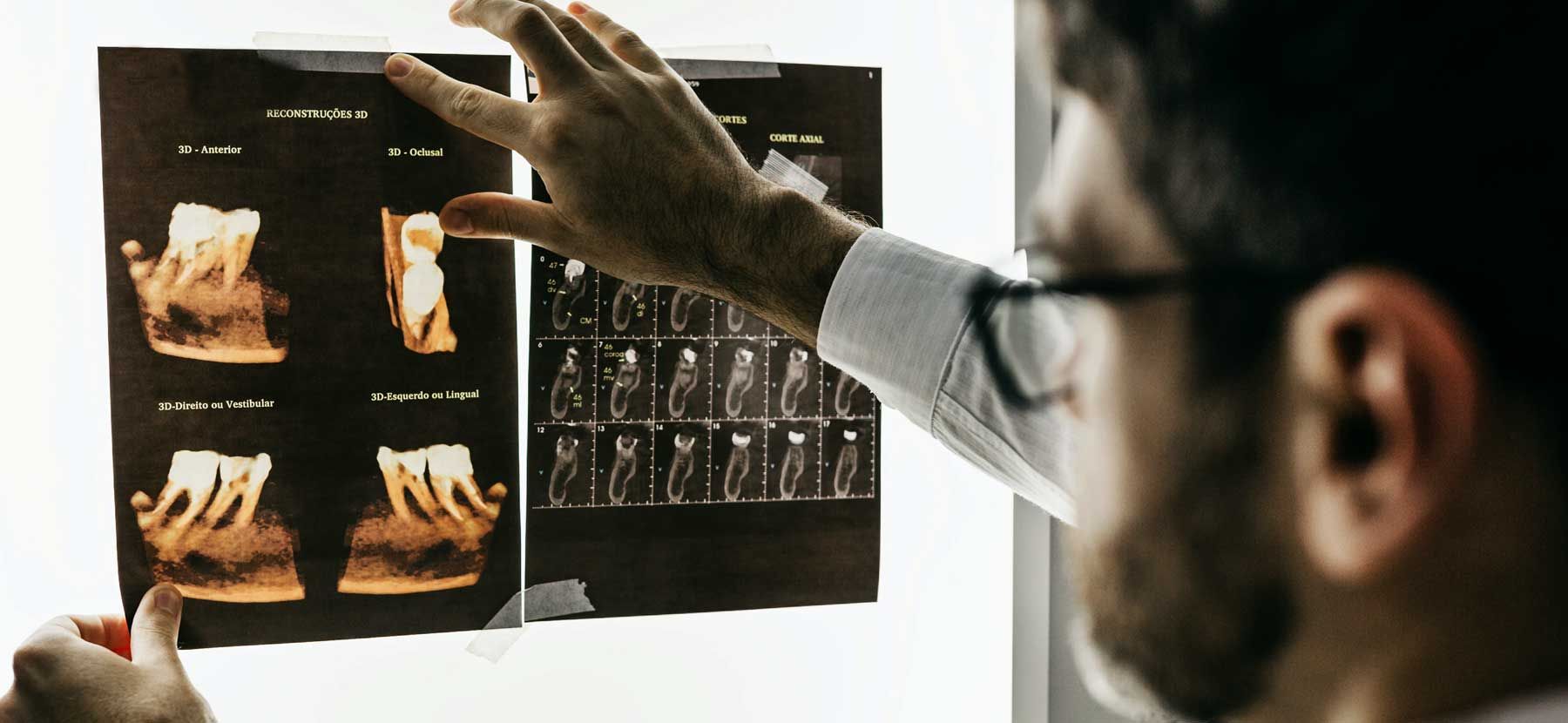
 WORRY FREE DIAGNOSIS: NON-TOXIC & NON-INVASIVE
WORRY FREE DIAGNOSIS: NON-TOXIC & NON-INVASIVENext-Gen Thermography
AlfaSight Thermometry directs you to ROOT CAUSE.
Opens your vision to Dental, Whole-Body,
and Breast dynamics objectively.
-
 state-of-the-art health testing system
state-of-the-art health testing systemThe AlfaSight 9000TM
AlfaSight allows you to
SEE ORGAN FUNCTION by utilizing the Autonomic Nervous System’s signaling by way of the segmental organ and tissue connections. -
 WHOLE-BODY WELLNESS SCAN
WHOLE-BODY WELLNESS SCANDetect Early Health Warnings
Add the AlfaSight 9000 to provide
SENSITIVE DIAGNOSTICS for prediction
and comprehension of disease processes. -
 WORRY FREE DIAGNOSIS: NON-TOXIC & NON-INVASIVE
WORRY FREE DIAGNOSIS: NON-TOXIC & NON-INVASIVENext-Gen Thermography
AlfaSight Thermometry directs you to ROOT CAUSE. Opens your vision to Dental, Whole-Body,and Breast dynamics objectively.
The AlfaSight 9000TM
FDA CLEARED
Dynamic Adjunct Diagnostics
A preventative health care device that helps identify subtle and influential physiological dysfunctions as well as identify hidden infections with specificity and sensitivity. AlfaSight 9000 is a state-of-the-art health testing system that highlights and prioritizes abnormalities of the tissues and organs key to disease contribution.
- Comprehensive analyses of temperature behaviors in the breast
- Neoplastic Conditions (identifies tumor-cellular environments)
- Inflammatory disorders and causal-factor identification
- Musculoskeletal disorders
- Extracranial cerebral and facial vascular disease
- Abnormalities of the thyroid gland
- Peripheral vascular disease
The AlfaSight 9000 Gives a
Comprehensive View of Dynamic Physiology
Comprehensive View of Dynamic Physiology
Discover insights and clarity as difficult cases are unfolded as patients are supported with early detection and true preventive care.
Dr. Daniel Beilin, founder and creator of the AlfaSight 9000 – partnered with leading clinics and physicians in integrative medicine in Europe for the past 30 years. Bringing this advanced technology to the U.S. and making it available to clinicians worldwide is revolutionizing the way integrative physicians approach patient care. The AlfaSight 9000 is an FDA (510k) cleared, cutting-edge adjunct diagnostic device that opens a health window to provide detection of physiological dysfunctions and contributions to the disease process
What Doctors are Saying...
-

Dr. Dietrich Klinghardt
MD, PhD, Integrative Medicine
UK & Washington, US."Alfa has taken thermography into the next century. We use it in my practice on every patient in order to not overlook the important underlying issues and this pattern recognition—which is really the combined work from many medical doctors—the knowledge is brought together. This pattern recognition is really something that doesn’t exist anywhere else in medicine, and so it’s a wonderful tool.”
-

Dr. Karin Nielsen
ND, Certified Clinical Thermographer
Baton Rouge, LA"Regulation Thermometry integrates and deepens the understanding I achieve when I use my infrared camera system on the breast. I can see distant influences and focal infections that can't be seen by any other method. I strongly endorse the AlfaSIght 9000 for any clinic, using with or without infrared camera systems.”"
-

Dr. Joseph Mercola
Osteopathic Physican, Best Selling Author, Chicago ILI want you to pay really careful attention to what Dan has to say. This technology is beyond extraordinary. My plea to each and every one of you is to get this equipment. This is the technology to use."
-

Dr. David Hickey
MD, Nuclear Medicine Physician
McKinney, TX“I am a Nuclear Medicine Physician and Radiologist and have been utilizing the technology for adjunct diagnostics called Regulation Thermography. It provides clear hints to identify the correct questions I should be asking the patient in order to establish a deeper, comprehensive view of disease and its complexities.”
-

Dr. Dawna Jones
MD, Alfa Practitioner
Norwell, MA“Regulation Thermometry allows me to identify and address underlying factors that play a role in women’s health, - especially breast health. This technology observes organ physiology dynamically and enables us to accurately address and target priority systems.. The benefits of Regulation Thermometry and the technology has proven itself to provide a calling to those who would like both preventive and highly effective strategies to health.”
-

Dr. Daniel Pompa
DC, Alfa Practitioner, Nutrition
Park City, UTThe AlfaSight 9000TM is an inexpensive and accurate test to determine cancer conditions years before you would get a conventional diagnosis."

![]() Alfa's founder Dr. Dan Beilin, talks about the future of medicine and how the AlfaSight 9000 can help your practice.
Alfa's founder Dr. Dan Beilin, talks about the future of medicine and how the AlfaSight 9000 can help your practice.
What is Regulation Thermometry?
Sampling dynamically-changing skin temperatures before and after a cool-air stress provides keys fulfilling known mathematical patterns that have been historically corroborated with laboratory and imaging diagnostics, backed by robust data accumulated over the last 40 years. Different from Infrared cameras (controversial in its medical applications), Regulation Thermometry utilizes the neurological aspects of disease reflection and utilizes artificial intelligence.
Regulation Thermometry is objective, scientifically-founded, and reproducible.

-
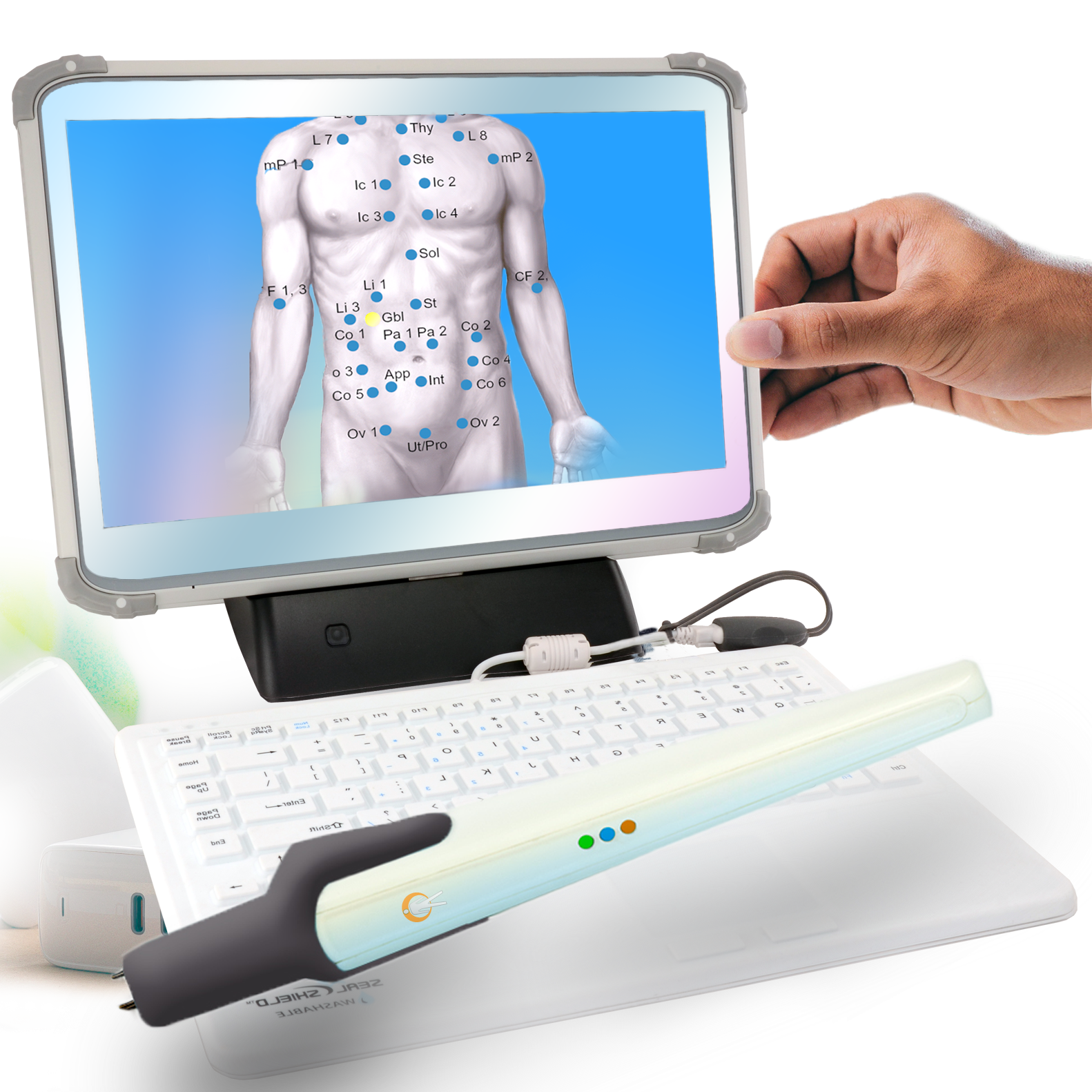
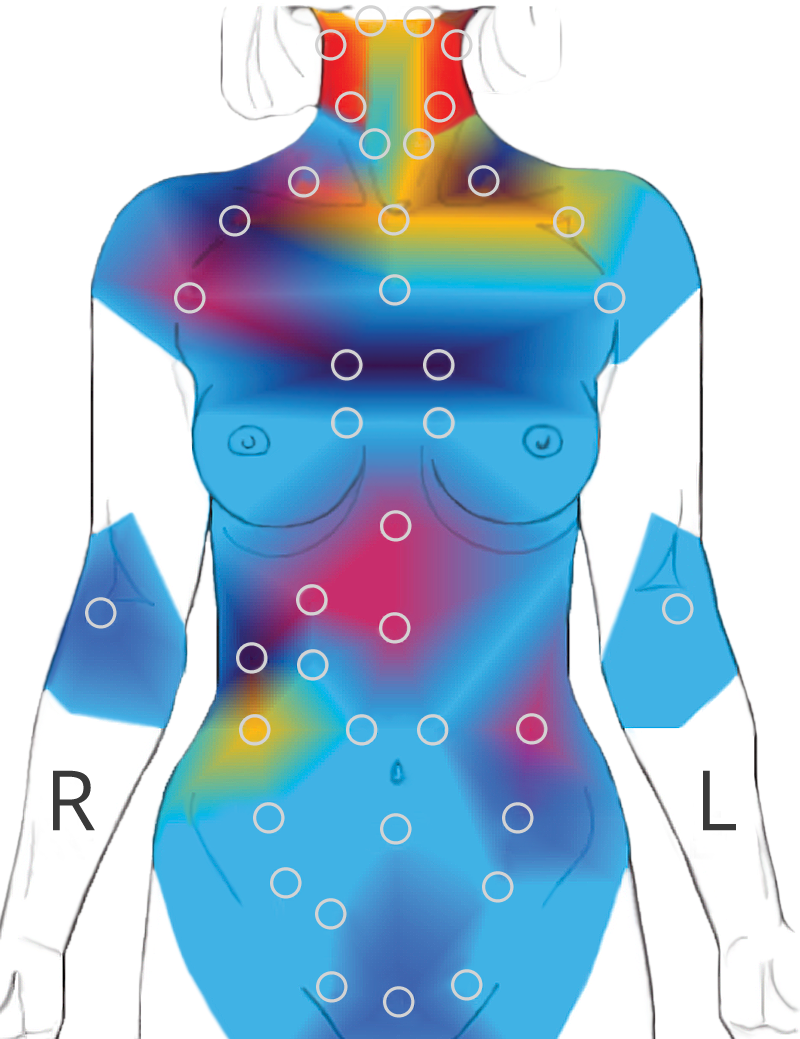
120 body points are tested with a non-contact infrared sensor. The test takes 20 minutes.
-
Reflects organ and tissue responses using the Autonomic Nervous System responses through the Spinal Reflex Arc.
-
Over 40 signature patterns are depicted and a multi-page report is delivered to the physician within seconds.
-
No radiation or discomfort to the patient.
Thermoregulation: The innate ability of the body to control its core temperature by shunting blood to the core or to the skin in order to achieve balance.
Thermometry: The digitized measurement of temperature. With the AlfaSight, points are sampled from the skin above various organ and nerve projection regions.
Thermography: A technique using a camera or sensor to attempt to identify pathological processes. Infrared cameras are mostly disregarded by radiologists but digitized behavior analytic methods as is used by the AlfaSight are receiving increasing respect due to its mathematical methods for analysis.
Team of Experts

Dr. Dan Beilin
Dr. Beilin has been named the most authoritative expert of Regulation Thermometry in the West, by the world expert Dr. Petra Blum. Dr. Beilin studied under Drs. Blum, Banis, Sauer, Klinghardt, and Rau and have discovered new algorithms for disorder identification now integrated into the AlfaSight reports. Dr. Beilin holds an OMD in Asian Medicine and a BSc. degree (Univ. Calif Davis) in Neurophysiology. Dr. Beilin has coauthored over 10 papers in Neurosurgical research and Gastroenterology. He is in private practice in California.

Dr. Thomas Rau
Dr. Thomas Rau is the medical director of one of the most famous Integrative Hospitals in the world, the Paracelsus Klinik, St. Gallen, Switzerland. Dr. Rau also a student of Dr. Blum has seen thousands of thermograms from the AlfaSight and highly respects its power to discern truly suspicious regions of the body that lead to corrective treatments. He currently owns 3 AlfaSight devices.

Jackie Bell
Jackie Bell has been part of the Alfa teaching team for 15 years. She is gifted in explaining the mechanisms and methods of interpretation as she had also studied under Dr. Blum and Dr. Beilin, as well as Dr. Rau. She is a valuable contributor to the ongoing benefits and publicity for this outstanding method.
Resources


Patients & Caregivers
Thousands of patients are safely tested for hidden factors in disease causes.
Find or tell your doctor!
Doctor Testimonial
Dr. Klinghardt on the importance of evaluating disease based on pattern identification by thermometry.
Articles
Alfa Worldwide
Better. Safer. More Accurate.
Non-Invasive and Non-Toxic.
Earth loves it.
The AlfaSight 9000TM is now in 23 countries with more coming online soon.

Find an Alfa Provider near you
USA: 866-652-6600
Alfa Headquarters
9057a Soquel Drive Suite B
Aptos, California USA 95003
Keep informed with Alfa News
THE ALFA NEWSLETTER WILL KEEP YOU IN TOUCH WITH THE LATEST PROGRESS, TECHNICAL UPDATES, ADVANCEMENTS AND MORE